New Orleans: Immunotherapy—The Game Is Still in Town
Quick Links
At the 33rd Annual Meeting of the Society for Neuroscience held last week in New Orleans, at least two dozen presentations dealt with current efforts to develop a vaccine for Alzheimer’s disease. This bold approach suffered a setback last year when meningoencephalitis in 6 percent of patients halted Elan/Wyeth’s (AN-1792 phase 2a trial), but it has since bounced back with a variety of approaches. Asked privately, a majority of scientists said they believe that vaccination will eventually work. These are among the most actively pursued questions: How best to avoid a harmful T cell response with clever design of Aβ construct and adjuvant choice; how well does amyloid clearance correlate with cognitive improvement; and is passive or active immunization the better way to go. Reflecting the ongoing pathophysiological debate about whether soluble oligomers/small aggregates or mature plaques do the most damage, some researchers place their bets more on targeting the former while others bank on clearing the latter. In truth, most researchers say that both sides overlap, and that perhaps a two-pronged approach could be found. Below are selected highlights.
Roger Nitsch at the University of Zurich recapped his group’s ongoing analysis of the still-blinded Zurich cohort of the AN-1792 trial (133.8). His data indicates that patients who formed antibodies declined less rapidly than those without a measurable immune response (see ARF related news story). Only some of the neuropsychological instruments used revealed a benefit; ADAS-Cog did not. The Zurich cohort had no plasma or CSF changes of Aβ. When grouped by the amount of antibody patients produced (high, medium, low), cognitive benefit correlated such that people with the highest titers tended to do best. This high-responder group included two patients who developed meningoencephalitis, Nitsch said. Overall, 18 people came down with the side effect, of whom 12 recovered within weeks but six remain with cognitive or neurological deficits, Nitsch added (see also Orgogozo et al., 2003). The side effect did not correlate with antibody titers, suggesting a T cell response.
MW Pride at Wyeth Research in Pearl River, New Jersey, with colleagues there and at Elan Biopharmaceuticals, reported on the cellular immune response by patients in the AN-1792 trial. The scientists isolated peripheral blood mononuclear cells (PBMCs) from patients in the phase 1 trial and phase 2a trials, and stimulated them with Aβ. Differences in the cytokine profile of the responding cells suggested that specific T cell responses elicited from phase 1 PBMCs were TH-2 biased, while those from the phase 2a were biased toward a proinflammatory TH1 response, the scientists write in their abstract. Both responses were directed against the C-terminus of Aβ, which is generally cut away in second-generation immunogens.
The reason for this differential response is still not entirely clear, but the adjuvant used, QS-21, is potent and known to elicit TH1-biased T cell responses, which can lead to autoimmune reactions.
David Cribbs of University of California, Irvine, with colleagues elsewhere, tried to redirect this TH1-response toward a presumably safer TH2-like response by using the adjuvant mannan. To enhance antigen presentation, Cribbs conjugated multiple copies of this group’s Aβ1-28 peptide to mannan in the form of “antigen trees.” Injecting small amounts of this into mice elicited responses directed at the first 15 amino acids of Aβ. The antibody types (mostly IgG) and cytokine profile indicated the response was biased toward TH2, Cribbs told the audience. To curtail the TH1 risk further, the team substituted the endogenous T cell epitope of Aβ with the synthetic T cell epitope PADRE (this is because a T cell activation is desirable to boost antibody titers, but need not be directed against Aβ/APP). This chimeric immunogen elicited high anti-Aβ antibody titers, yet T cells from mice immunized with it fail to respond to Aβ or APP, Cribbs said (133.6).
Hideo Hara of the National Institute of Longevity Sciences in Obu City, Japan, presented initial data on an oral vaccine that decreased amyloid deposits in transgenic mice without any adjuvant, nor with an apparent T cell response. This approach packages Aβ into an AAV vector and stimulates a mild humoral response via Aβ presentation to the mucosal immune system in the intestine, Hara reported (201.2).
One major question in the field concerns the role of microglia (see related New Orleans news story). Are they key to the removal of amyloid? Prior work by scientists at Elan and elsewhere had suggested that the Fc tails of Aβ antibodies bind Fc receptors on microglia, which activates them to ingest amyloid deposits. This is widely considered a major mechanism of amyloid clearance, and Donna Wilcock, with David Morgan and colleagues at University of South Florida, Tampa, developed an ingenious way to test it (133.1). She injected either full-length Aβ antibodies or F(ab)2 fragments lacking an Fc domain into frontal cortex or hippocampus of transgenic mice, and then suppressed microglial activation pharmacologically with dexamethasone, a new NSAID, or the antibiotic minocycline. The more these drugs dampened microglial activation, the less compact amyloid was removed near the injection site. F(ab)2 fragments were unable to activate microglia and were similarly inept at getting compact amyloid removed. Intriguingly, however, the fragments did clear diffuse deposits quite efficiently, suggesting that different mechanisms could be harnessed for the removal of mature plaques and the soluble pool. The work also suggests, though, that NSAIDs that inhibit microglial activation might undercut the effect of a vaccine and should perhaps not be administered simultaneously, the researchers said. The study is in press at Neurobiology of Disease.
A simple yet beautiful demonstration of how neurons perk up once plaques are gone came from Julianne Lombardo, working with Brian Bacskai and Brad Hyman at Massachusetts General Hospital in Charlestown. Neuronal dendrites that grow through and around plaques are known to look abnormal: Rather than growing straight through the neuronal parenchyma, these neurites are curvy and strangely swollen. Could clearing plaques restore their morphology, or is such a brain beyond repair? To address this question, the researchers injected a single dose of antibody into the cortex of PDAPP mice, sacrificed them, and examined their brains with immunohistochemistry. After four days, and lasting for about a month, amyloid plaques disappeared and, amazingly, the neurites simply straightened themselves out once the obstacles were gone. This experiment did not address corresponding behavioral benefits, and whether the aging human brain would right itself similarly is an open question. Still, the result suggests that the brain may be able to correct itself after a passive immunotherapy, and it extends a growing understanding of the plasticity of the adult brain. This work is in press at J. Neuroscience.
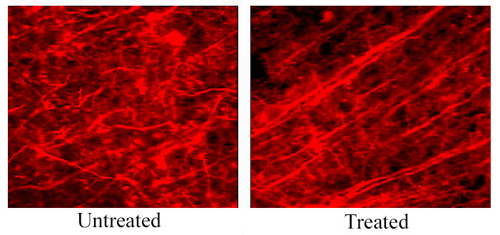
These images show SMI32 (anti-neurofilament) staining in the brain of a PDAPP mouse. The left side shows neurites in an untreated area of the cortex, away from where the antibody was applied. There is a large amyloid plaque within this field (not shown), and numerous distorted, curvy neurites. The right side shows a field of neurites within the 10d5 anti-A& antibody treatment area, showing neurites with normal, straight trajectories. This surprising "restoration" of distorted neurites occurs within four days after antibody treatment, and lasts at least up to 1 month.
A variety of different vaccination approaches clear amyloid and improve performance of various models. Elan and Wyeth scientists led by Peter (133.3) compared full-length Aβ1-42 with the amino-terminal fragment Aβ1-7, and passive immunization with monoclonals either against plaques (the 3D6) or against soluble Aβ (the m266 antibody). The Elan group still considers vaccines that trigger microglial phagocytosis most efficient, as both passive administration of 3D6 and active treatment with the small N-terminal Aβ fragment cleared amyloid pathology. A second presentation by Elan scientists (201.13) showed that AD patients in the AN-1792 trial had produced antibodies mainly against the first nine amino-terminal residues of Aβ, again suggesting the peptide’s C-terminus is dispensable for a strong humoral response.
As expected, the m266 antibody did not clear plaque deposits; this implies that rapid improvements in a learning task seen with this antibody occur because it interferes with acute effects of soluble Aβ, perhaps on synapses (see ARF related news story). Other scientists speculated that soluble Aβ might explain the day-to-day variations in cognition frequently seen in AD patients, rather than the gradual decline over time. Several groups presented work on antibodies which appear to be oligomer-specific and could remove soluble pools of Aβ. While such novel antibodies could yield rapid effects on cognition, the studies have not yet clearly demonstrated that these antibodies are specific to Aβ. Further research must show that they don’t cause problems by interacting with other physiological proteins that can oligomerize, such as insulin.
The m266 antibody has gained fame also because it fueled the peripheral sink hypothesis of AD vaccination, which holds out hope that passive immunization could “draw” out brain Aβ (see ARF related news story). This study raised a question about the staying power of this effect; in other words, can plasma Aβ increases after an antibody injection really predict future decreases in brain Aβ? Addressing this issue was a longitudinal study by the research collaboration between Steven Paul, Ron DeMattos at Eli Lilly and Company in Indianapolis, Dave Holtzman at Washington University, St. Louis, and colleagues. The scientists injected m266 into APPV717F mice at four and eight months of age, measured their plasma Aβ levels the next day, let them age, and at 12 months quantified their amyloid burden immunohistochemically. Results differed somewhat between Aβ42 and 40, but overall, the scientists reported that relative increases in plasma Aβ soon after immunization indeed correlated with, i.e., predicted, amyloid burden in the hippocampus and cortex at an older age (842.19).
Pritam Das and VG Howard, working with Todd Golde at the Mayo Clinic in Jacksonville, Florida, reported that passive immunization with an unusual antibody that recognizes the C-terminal end of soluble Aβ (most antibodies bind the other end) effectively reduced brain levels of Aβ40 and 42, while increasing plasma levels 25-fold. An ongoing longitudinal study will address cognitive effects, if any, pathology, and possible mechanisms (133.13).
Cindy Lemere’s group at Brigham and Women’s Hospital, Boston, presented the latest data on their immunization of the Caribbean vervet monkeys, following her presentation at last year’s CSH meeting (scroll to Lemere in ARF related news story). There, Lemere had reported high antibody titers and reductions in CSF Aβ after immunizing five aged monkeys with Aβ42. In New Orleans, Lemere reported that brain levels of insoluble Aβ42 were reduced by two-thirds in the immunized monkeys, while soluble Aβ40 levels were unchanged. Brains of immunized monkeys contained no Aβ42-immunoreactive plaques in six regions checked, but controls did (133.5). These results recapitulate results in mice and make this non-human primate an alternative model for vaccine development and other AD studies. The monkeys contained no tangles but did have hyperphosphorylated tau in neurites near plaques, Lemere said. The next immunization study will assess cognition in the monkeys before and after treatment. Lemere’s lab also presented characterizations of the mouse immune response following intranasal vaccination, and reported that genetic background and choice of adjuvant can greatly influence the nature of the immune response to immunization with Aβ and its peptide fragments (201.1, 201.11), suggesting that choice of adjuvant may be key in developing the human vaccine, too (see also Furlan et al., 2003).
Einar Sigurdsson and colleagues at New York University School of Medicine reported that a modified version of their K6Aβ1-30 vaccine (see ARF related news story), in which a hydrophobic region around amino acid 18 and 19 was altered, improved the performance of Tg2576 mice in the radial arm maze, even though antibody titers were low and the amyloid burden shrank only modestly, affecting only small plaques. The work suggests that massive amyloid clearance and high titers may not be necessary for a therapeutic effect (133.10).
Though approaches vary, it appears that the strongest benefit to date lies in preventing future deposition and mild cognitive improvement in older animals with severe deposition; neuritic plaques generally stay in place. No new trials were officially announced, though speculation was rampant. This news summary can’t be comprehensive. My apologies to all whose work went unmentioned; as always, I encourage additions and corrections. You can view abstracts mentioned in this story at the SfN/ScholarOne website.—Gabrielle Strobel.
References
News Citations
- Alzheimer’s Vaccine: In Some Patients, at Least, It Might Just Work
- New Orleans: New Approaches to Lift Microglia Mysteries
- One-Shot Deal? Mice Regain Memory Day After Vaccination, Plaques Stay Put
- Early Diagnosis of Alzheimer's—Making Use of the Blood-Brain Barrier
- Budding RNAi Therapies, APP Protein Interaction Map Impress at Meeting
- A Kinder, Gentler Alzheimer's Vaccine?
Paper Citations
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003 Jul 8;61(1):46-54. PubMed.
- Furlan R, Brambilla E, Sanvito F, Roccatagliata L, Olivieri S, Bergami A, Pluchino S, Uccelli A, Comi G, Martino G. Vaccination with amyloid-beta peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain. 2003 Feb;126(Pt 2):285-91. PubMed.
Other Citations
External Citations
Further Reading
News
- MHC Polymorphisms Influence Immune Response to Amyloid β
- Trials and Tribulations—Autopsy Reveals Pros and Cons of AD Vaccine
- Following Footsteps of AD Vaccination: Passive Shots Against Prions Protect Mice
- Pertussis Toxin Stokes Autoimmune Reaction in Aβ-Vaccinated Mice
- Plaque Clearance, Antibody Isotype Are Key for Passive Aβ Immunization
Annotate
To make an annotation you must Login or Register.

Comments
�
I am really happy that science is going to immunotherapy of Alzheimer's disease. I presented some of my data at the 6th International Conference on Alzheimer's Disease and Related Disorders, July 1998 in Amsterdam, The Netherlands, and this data is published. When I presented disappearing diffuse amyloid plaques in brain in my experimental model and proposed as a mechanism that they probably were disappearing because of "possible immunization," the leading scientists in the Alzheimer's disease arena did not believe me. Next I published a full paper in NeuroReport. In the discussion I write, "Probably antibodies against amyloid might act as an artificial chaperone for extra- and intracellular amyloid. Our data raise the possibility of vaccination with amyloid against AD (Alzheimer's disease)." In conclusion: "Collectively, the results raise the possibility that frequent injection with amyloid may be sufficient in preventing and treating the development of amyloid plaque formation in AD (Alzheimer's disease)."
References:
Pluta R, Barcikowska M, Mossakowski MJ, Zelman I. Cerebral accumulation of beta-amyloid following ischemic brain injury with long-term survival. Acta Neurochir Suppl. 1998;71:206-8. PubMed.
Pluta R, Barcikowska M, Misicka A, Lipkowski AW, Spisacka S, Januszewski S. Ischemic rats as a model in the study of the neurobiological role of human beta-amyloid peptide. Time-dependent disappearing diffuse amyloid plaques in brain. Neuroreport. 1999 Nov 26;10(17):3615-9. PubMed.
Make a Comment
To make a comment you must login or register.