Plasma p-Tau-217 Assays Work Well, But No Home Run for Diagnosis
Quick Links
Feel like your head is spinning? Like a little blood sample in a centrifuge, perhaps? No need to panic. It just means you are straining, like the rest of us, to keep up with the Alzheimer's disease plasma biomarker development field. New markers, new assays, new protocols seem to arrive at a dizzying pace. A flurry of those was on display at this year’s CTAD meeting, held last month in Boston. “The field is moving very rapidly,” noted Andreas Jeromin, ALZpath Inc.
- In round-robin, p-tau217 flies into the lead.
- Accuracies fall just shy of what’s needed for a stand-alone test.
- Using two cut points could close the gap.
Scientists reported results of a round-robin comparison of more than two dozen plasma phospho-tau assays. (Hint: p-tau 217 tests outdid the rest of the flock.) A clearer picture emerged of how plasma markers might be used for diagnosis. (Another hint: Two cut points work better than one). Plus attendees saw early data from two new commercial tests, ALZpath’s p-tau 217 assay and PrecivityAD2 (see Part 12 of this series)..
Straight Answers from a Round-Robin?
Nicholas Ashton, University of Gothenburg, Sweden, presented results of the round-robin of the Global Biomarker Standardization Consortium, an initiative originally started by the Alzheimer’s Association to standardize and validate biomarker tests. The GBSC had previously reported poor correlation among 11 different plasma Aβ42/40 assays conducted at 11 different sites (Aug 2019 conference news; Pannee et al., 2021). At least for p-tau217, the picture now looks a little rosier.
Ashton and colleagues compared 26 different p-tau tests by 12 different vendors, 11 commercial ones plus UGot. Participation was free of charge, but also blinded, meaning no handler knew who the samples came from, and no vendor knew how any other vendors’ assays performed. They joined anyway. “The response was remarkable,” Ashton told Alzforum. Vendors included AbbVie, ADx Neurosciences, Alamar, ALZpath, Fujirebio, Janssen, Lilly, MagQ, Meso Scale Diagnostics, Roche, and Quanterix (image below). An exception was C2N Diagnostics, St. Louis, which was invited to participate but did not.
For the test samples, Jonathan Schott, University College London, provided Ashton with 1 mL of plasma from each of 40 individuals with suspected AD. It turned out that 25 of these had AD, based on cerebrospinal fluid Aβ42/40 and p-tau181. Ashton aliquoted their plasma into smaller samples he shared with each participating lab. Each then determined who among the 40 donors likely had AD, and who didn’t, based on plasma p-tau181, p-tau212, p-tau217, or p-tau231. Accuracies were determined by the area under the curve, or AUC, of sensitivity versus specificity plots—an AUC of 1.0 being perfection and 0.95 or better typically deemed highly desirable for routine clinical work.
Most of the 26 assays identified AD donors from controls with high accuracy (image below). Among them P-tau217 stood out for consistently high AUCs, and a greater fold increase between AD and controls. Ashton acknowledged that the sample size of 40 was small. That said, he was encouraged by the tight agreement among the 11 p-tau217 tests—between-assay correlations ran from 0.85 to 0.97.
“This gives us hope that the field does not have to decide on one [p-tau217] assay, but can convert between them,” said Ashton. “This suggests that it is possible to combine and interpret data from large research cohorts, or from different clinical trials, that are based on different assays,” he added. The Alzheimer's fluid biomarker field has long struggled with such comparisons because of the number of different tests in use.
Ashton also noted that immunoassays running on Fujirebio's Lumipulse readers emerged on top, performing on par with a mass spectrometry test run by U Gothenburg. This is good news for the field because Lumipulse machines are already being widely used in clinical practice, often being the diagnostic workhorse in hospitals. They are fully automated, meaning the sample never gets processed by human hand. They are also fast, taking about 20 minutes to run a test. “This is what the future of AD clinical testing will look like,” predicted Ashton.
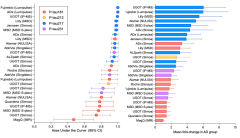
P-tau217 Wins Round 1. In a round-robin analysis of 40 plasma samples, tests for p-tau217 best distinguished people with AD from healthy controls (left). They also record the biggest fold differences (right). [Courtesy Nick Ashton, University of Gothenburg.]
P-tau181 and p-tau231 also performed well. Correlations among the 10 p-tau181 assays ranged from a low of 0.04 to 0.9. Ashton puts this down partly to the assays and partly to plasma. Unlike p-tau217, which can only be detected in plasma after amyloid pathology has begun, p-tau181 and p-tau231 are found in plasma of healthy controls. “There seems to be a basal level of noise that accounts for some of the variability,” said Ashton. AUCs for p-tau231 assays ranged from about 0.8 to about 0.95, but correlations between assays were not shown, perhaps because there were only four of them.
Finally, one assay from UGot of p-tau212 predicted AD with an AUC of about 0.9. It is too soon to pit the accuracy of this relative newcomer against that for p-tau217, but p-tau212 might have an edge because it may be one of the earliest markers of amyloid pathology.
To Ashton’s mind, a caveat emerged from the round-robin. That is, correlations between plasma and CSF were not terribly strong for any given marker. For Fujirebio’s p-tau217 test, the correlation coefficient was 0.67; for U Gothenburg’s test, only 0.48. “This is a really important take-home message,” said Ashton. “Once you get into the positive range, the correlation is almost nonexistent.”
He said this should be a consideration for the proposed new NIA-AA diagnostic criteria, which lump plasma and CSF together to predict the initial stage of AD (Aug 2023 news). “I don’t think this is the way to go,” said Ashton. “You cannot directly stage people when mixing plasma and CSF,” he said, though he said other assays might have tighter correlations.
Suzanne Schindler, Washington University, St. Louis, noted as much for assays that use the p-tau217/tau217 ratio instead of p-tau217 absolute values. She told Alzforum that among 529 people studied at the Knight Alzheimer’s Disease Research Center, the correlation between the percentage of p-tau217/tau217 in plasma and CSF, as measured by mass spectrometry, was about 0.85. Scientists hope that measuring the percentage phosphorylation normalizes for disconnects in the plasma/CSF equilibrium caused by worsening pathology or by co-morbidities. In effect, the non-phosphorylated tau would act as an internal standard.
Research Versus Implementation
In her CTAD talk, Schindler emphasized that some of the blood-based biomarker tests, particularly those for p-tau217, are now as accurate as CSF tests. “We are at the point where we need to think about whether we even need to follow up blood tests with CSF or PET,” she said. Still, she thinks that even with AUCs of 0.95, the tests are not good enough. In a clinical population where 61 percent are amyloid-positive, a typical scenario at memory clinics, such tests would still misclassify one in 10 people, she calculated.
From a treatment perspective, this would not work, agreed Oskar Hansson, Lund University, Sweden. The problem, Hansson told Alzforum, is that shifting from treating markers as continuous variables, as scientists do in research settings, to a yes/no diagnostic requires too much simplification of the data. “The values that are close to the yes/no cutoff are always uncertain,” he said.
He sees a solution in setting two cutoffs. Values higher than the top cutoff would identify people who are sure to be positive, and values lower than the bottom cutoff would distinguish those sure to be negative. Anyone who fell in between could be sent for further testing. “Clinics are doing that for prostate specific antigen tests right now,” Hansson said. Men whose tests are uncertain go for follow-up MRI scans.
At CTAD, Hansson showed how this approach could work for AD. Collaborating with Nicolas Barthelemy and colleagues at WashU, who have perfected an MS-based assay to measure %p-tau217 in plasma, the scientist tested samples from the Swedish BioFinder2 study and the Knight ADRC at Washington University, scientists in his lab were able to bump up the test's positive and negative predictive values by using two cutoffs. About 20 percent of the cohort fell into the uncertain zone. Indeed, these people had less amyloid plaque as seen on PET scans than the clear p-tau217 positives.
The strategy also worked for identifying people with neurofibrillary tangles. Basically, the two-cutoff approach upped both the positive and the negative predictive values from 90 to 95 percent, the magic threshold clinicians want for a practical diagnostic test. Hansson and colleagues saw similar improvements in diagnostic performance of Lilly and Janssen's p-tau217 plasma assays when they used two cutoffs (Brum et al., 2023).
Schindler said she likes this approach. In clinical practice, however, she envisions two tests being used: a triage test and a confirmatory test. The former would identify people who likely have AD, but would need to be followed up with amyloid PET, especially if the person were going to receive immunotherapy based on the outcome. The confirmatory test would have to be good enough to stand alone.
A blood-based biomarker working group convened by George Vradenburg at UsAgainstAlzheimer’s recommended the triage and confirmatory tests. The group’s goal is to speed up and facilitate treatment of AD, said Schindler, who is a member.
How is a triage test useful if it requires a follow-up test? For one, it could help clinicians figure out who goes to a specialist. “Dementia clinics are inundated, and it can often take a year or more to get an appointment,” said Schindler. “A blood test could help us identify who needs to be seen soonest.” For another, a triage test could rule out people who do not have AD. It would need to be highly sensitive. “In populations where the prevalence of AD is low, a negative result is almost certainly negative if the test has high negative predictive value,” said Schindler. “We think a triage test will be helpful for people who are ‘worried well.' Then we could look for other explanations for their memory concerns," she told Alzforum. For more on the newest p-tau217 tests, see the next story in this series.—Tom Fagan
References
News Citations
- Two New p-Tau217 Blood Tests Join a Crowded Field
- Are Aβ Blood Tests Ready for Prime Time?
- Revised Again: Alzheimer's Diagnostic Criteria Get Another Makeover
Paper Citations
- Pannee J, Shaw LM, Korecka M, Waligorska T, Teunissen CE, Stoops E, Vanderstichele HM, Mauroo K, Verberk IM, Keshavan A, Pesini P, Sarasa L, Pascual-Lucas M, Fandos N, Allué JA, Portelius E, Andreasson U, Yoda R, Nakamura A, Kaneko N, Yang SY, Liu HC, Palme S, Bittner T, Mawuenyega KG, Ovod V, Bollinger J, Bateman RJ, Li Y, Dage JL, Stomrud E, Hansson O, Schott JM, Blennow K, Zetterberg H. The global Alzheimer's Association round robin study on plasma amyloid β methods. Alzheimers Dement (Amst). 2021;13(1):e12242. Epub 2021 Oct 14 PubMed.
- Brum WS, Cullen NC, Janelidze S, Ashton NJ, Zimmer ER, Therriault J, Benedet AL, Rahmouni N, Tissot C, Stevenson J, Servaes S, Triana-Baltzer G, Kolb HC, Palmqvist S, Stomrud E, Rosa-Neto P, Blennow K, Hansson O. A two-step workflow based on plasma p-tau217 to screen for amyloid β positivity with further confirmatory testing only in uncertain cases. Nat Aging. 2023 Sep;3(9):1079-1090. Epub 2023 Aug 31 PubMed.
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.