Glial Cells Refine Neural Circuits
Quick Links
Part one of a two-part story. Read part two here.
Neurodegenerative disease researchers have caught on that glia do more than supply energy to neurons or respond to emergencies such as amyloid accumulation or tissue damage. At the Society for Neuroscience (SfN) annual meeting, which drew nearly 25,000 scientists to San Diego November 9–14, researchers reported new data on the fundamental roles played by glial cells in forming and refining neural circuits. As the brain ages, those functions seem to get creaky, raising the possibility that keeping glial cells young and fit could protect against neurodegeneration. Other scientists outlined new approaches to promote a kind of glial cell activation that might help mice tackle Alzheimer’s-like pathology in the brain (see part two of this series).
Won-suk Chung, a postdoc in in Ben Barres' lab at Stanford School of Medicine, Palo Alto, California, studies astrocytes—the most common glial cell in the brain. Earlier work in the lab had revealed that the star-shaped cells express high levels of phagocytic receptors. Given that an individual astrocyte can touch thousands of synapses, Chung and colleagues wondered if astrocyte phagocytosis contributes to synaptic turnover. As described on their poster—and reported 24 November in Nature—they first examined neuronal-glial interactions in mice less than a week old. At this young age, retinal ganglion cells shed synapses as a normal part of visual system development. Using confocal microscopy, the researchers saw astrocytes engulfing axon terminals and synapses on these retinal cells. They saw the same with array tomography, a newer technique that allows quantitative, high-resolution imaging of serial tissue sections (see Micheva and Smith, 2007).
To test whether astrocyte phagocytosis is responsible for this synaptic pruning, Chung turned to mice lacking multiple EGF-like-domains 10 (MEGF10) and C-mer proto-oncogene tyrosine kinase (MERTK), two phagocytic receptors that are highly expressed in astrocytes (see Jan 2008 news story). Unlike wild-type cells, astrocytes in the MEGF10- and MERTK-deficient mice ingested no synaptic components and the visual system of these animals failed to mature, suggesting that phagocytosis of synapses contributes to development of the retina.
The wild-type astrocytes phagocytosed synapses in 1- and 4-month-old mice, as well, indicating that they prune synapses into adulthood. Based on preliminary data in older mice, however, Barres suspects the rate by which astrocytes eat synapses slows with age, leading to accrual of senescent synapses that would normally get recycled. If this in fact occurs, Barres said, it would explain the dramatic rise in complement component C1q detected at synapses in the brains of wild-type mice and people during normal aging (see Stephan et al., 2013). C1q is a lectin-like protein that binds apoptotic cells. “We assume that it binds senescent synapses in the aging brain,” Barres said. He plans to examine AD mouse models to see if their astrocytes engulf synapses poorly relative to wild-type animals. Prior work suggested complement helps rid plaques in AD mice (see Jun 2008 news story), though perhaps at the expense of cognition (Aug 2013 news story).
Marie-Ève Tremblayof Laval University, Quebec City, has addressed similar questions in her studies of how microglia interact with synapses. Using two-photon imaging, electron microscopy, and array tomography, she and others have reported that microglia cozy up to synapses and then chew them up in the brains of early postnatal and adolescent wild-type mice (see Nov 2010 news story; Schafer et al., 2012). However, in older mice, which lose cortical sensory neurons during age-related vision and hearing loss, Tremblay found that microglia are misshapen and clump together instead of spreading evenly throughout the brain as they do in younger animals (Tremblay et al., 2012). The findings agree with another two-photon imaging study that showed aging microglia phagocytosing more slowly and responding to tissue injury poorly compared to microglia in young mice (see Hefendehl et al., 2013), Tremblay said.
What about in mice modeling Alzheimer’s disease (AD)? Something similar seems to happen, Tremblay said. When her team examined brains of 6-month-old APP/PS1 mice with extensive plaque deposition, they saw that phagocytic capacity was down—APP/PS1 microglia interacted with synapses less and engulfed them only half as often as microglia from age-matched wild-type littermates (see image below). Comparing microglia from the transgenic mice to aged wild-type microglia, “what is similar is that microglia are impaired in their ability to phagocytose,” she said. “However, in wild-type animals you don’t see the problem until 20 months of age, whereas in APP/PS1 mice it appears at six months.”

Less appetite.
Microglia (purple) in the hippocampus of wild-type mice chomp synapses (uncolored inclusions) heartily (left image), microglia from age-matched APP/PS1 mice much less so (right image). Image courtesy of Marie-Ève Tremblay.
The findings suggest to Tremblay that microglia might contribute to AD by phagocytosing too little rather than too much. Consistent with this notion, a recent study found that cultured microglia from AD mice were slower than wild-type microglia at phagocytosing fluorescent beads. The most dysfunctional microglia came from brain areas laden with Aβ, and relieving plaque load with anti-Ab antibodies restored the glial cells’ phagocytic capacity (Krabbe et al., 2013).
Other researchers at the meeting asked about data on human microglia. Tremblay said it is hard to find human tissue samples of sufficient quality for this type of analysis. She told Alzforum her team plans to collaborate with Naguib Mechawar of the Douglas-Bell Canada Brain Bank in Montreal to analyze human AD brain for phagocytic defects.
Two-photon imaging data on an SfN poster by Jean-Philippe Michaud and Serge Rivest of Laval University support the idea that innate immune cells become sluggish with age. The researchers analyzed APP/PS1 mice whose monocytes express green fluorescent protein. Imaging through a 1-mm cortical window each week from 4 to 9 months of age, the researchers watched GFP-labeled monocytes clearing Aβ out of veins that permeate the cerebral cortex (see image below).
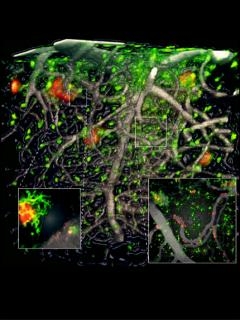
Scavengers in action.
Live imaging by two-photon microscopy shows monocytes (green) clearing Aβ (red) from veins (grey) in the cerebral cortex of transgenic mice modeling AD. Image courtesy of Paul Préfontaine and Jean-Philippe Michaud.
In 4-month-old AD mice with normal cognition despite accumulating plaques, “there seemed to be a natural defense mechanism where monocytes were able to clear vascular Aβ,” Rivest told Alzforum. However, by 9 nine months of age, this clearance was slowing down. The findings were published in the November 14 Cell Reports. In the future, the researchers hope to identify the molecular mechanisms that enable monocytes to home to vascular amyloid. Transcriptional profiling of monocytes from AD patients at different disease stages may help with this goal, Rivest said (see also part two).
However, disease can also correlate with overactive microglia. In a recent analysis of mice modeling human immunodeficiency virus-associated neurocognitive disorder, Tremblay and colleagues reported that cognitively impaired animals have overly phagocytic microglia, compared with wild-type littermates. A small-molecule phagocytosis inhibitor slowed dendritic spine loss in the disease mice; in vitro, it protected neurons against phagocytosis by microglia from these animals (see Marker et al., 2013).
Besides chewing things up—whether synapses, amyloid or other debris—activated microglia also release trophic factors that help build connections. At SfN, two-photon imaging data on a poster by Akiko Miyamoto and Junichi Nabekura of the National Institute for Physiological Sciences, Okazaki, Japan, showed that microglia are required for dendritic spines to form in the somatosensory cortex of early postnatal mice. Taken together, the data from the Canadian and Japanese groups suggests that microglia not only break down synapses but also help make new ones, adding synapse formation to the list of potential mechanisms that go awry in aging and AD.—Esther Landhuis
References
News Citations
- Innate Immune Cells Enlisted to Clear Amyloid, Fight Disease
- Not Just “Glia”: Astrocytes Are Specialized Eating Machines, Not Oligodendrocyte Siblings
- Complement: AD Friend or Foe? New Work Tips Balance to Former
- Curbing Innate Immunity Boosts Synapses, Cognition
- No Rest for Microglia: These Immune Cells Manage Healthy Synapses
Paper Citations
- Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007 Jul 5;55(1):25-36. PubMed.
- Stephan AH, Madison DV, Mateos JM, Fraser DA, Lovelett EA, Coutellier L, Kim L, Tsai HH, Huang EJ, Rowitch DH, Berns DS, Tenner AJ, Shamloo M, Barres BA. A Dramatic Increase of C1q Protein in the CNS during Normal Aging. J Neurosci. 2013 Aug 14;33(33):13460-74. PubMed.
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012 May 24;74(4):691-705. PubMed.
- Tremblay MÈ, Zettel ML, Ison JR, Allen PD, Majewska AK. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia. 2012 Apr;60(4):541-58. PubMed.
- Hefendehl JK, Neher JJ, Sühs RB, Kohsaka S, Skodras A, Jucker M. Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell. 2014 Feb;13(1):60-9. Epub 2013 Sep 18 PubMed.
- Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, Miller KR, Prokop S, Kettenmann H, Heppner FL. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8(4):e60921. PubMed.
- Marker DF, Tremblay MÈ, Puccini JM, Barbieri J, Gantz Marker MA, Loweth CJ, Muly EC, Lu SM, Goodfellow VS, Dewhurst S, Gelbard HA. The new small-molecule mixed-lineage kinase 3 inhibitor URMC-099 is neuroprotective and anti-inflammatory in models of human immunodeficiency virus-associated neurocognitive disorders. J Neurosci. 2013 Jun 12;33(24):9998-10010. PubMed.
Further Reading
Papers
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007 Dec 14;131(6):1164-78. PubMed.
- Stephan AH, Madison DV, Mateos JM, Fraser DA, Lovelett EA, Coutellier L, Kim L, Tsai HH, Huang EJ, Rowitch DH, Berns DS, Tenner AJ, Shamloo M, Barres BA. A Dramatic Increase of C1q Protein in the CNS during Normal Aging. J Neurosci. 2013 Aug 14;33(33):13460-74. PubMed.
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012 May 24;74(4):691-705. PubMed.
- Tremblay MÈ, Zettel ML, Ison JR, Allen PD, Majewska AK. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia. 2012 Apr;60(4):541-58. PubMed.
- Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, Miller KR, Prokop S, Kettenmann H, Heppner FL. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8(4):e60921. PubMed.
Primary Papers
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013 Dec 19;504(7480):394-400. Epub 2013 Nov 24 PubMed.
- Michaud JP, Bellavance MA, Préfontaine P, Rivest S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 2013 Nov 14;5(3):646-53. Epub 2013 Nov 7 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.