Antisense Therapy Stifles CSF Tau in Mild Alzheimer’s Disease
Quick Links
While many players in the tau therapy field are banking on immunotherapies to clear neurofibrillary tangles from the brain, some are focused on stopping tau production. In a poster presented at the Alzheimer’s Association International Conference, held July 26-30 in Denver and online, Catherine Mummery, University College London, Candice Junge, Ionis Pharmaceuticals, Carlsbad, California, and colleagues gave a peek at data from the first-ever trial of a tau antisense oligonucleotide in mild Alzheimer’s disease. In this Phase 1b trial, BIIB080, developed by Ionis in partnership with Biogen Inc., Cambridge, Massachusetts, caused no serious adverse events. It did reduce both total tau and phosphotau-181 in the cerebrospinal fluid by 30 to 50 percent. What this means for cognition remains unclear. A Phase 2 trial is planned to begin in mid-2022.
- In Phase 1 trial, tau antisense therapy appeared safe.
- BIIB080 lowered CSF tau levels by 30 to 50 percent.
- Phase 2 planned to start next year.
“This is a landmark study of human tau therapeutics,” Adam Boxer, University of California, San Francisco, told Alzforum. “It is the first to target tau at the genetic level and shows that, in a small number of people for a relatively limited duration, a significant reduction of tau protein in CSF is safe and well-tolerated.” Gil Rabinovici, also at UCSF, agreed, noting that “ASOs are a very promising potential approach to treating tauopathies.”
Antisense oligonucleotides match to specific snippets of mRNA, suppressing translation. They have seen a revival, spurred in part by the approval of Ionis’ nursinersen for spinal muscular atrophy (Nov 2016 news; May 2018 conference news). ASOs are being tested for their potential as therapeutics in neurodegenerative diseases, including AD (Aug 2019 news).
For this Phase 1b study, researchers recruited 46 people ages 50 to 74 with mild AD from the U.K., Canada, Germany, Sweden, Finland, and the Netherlands. AD diagnosis was determined by an overall Clinical Dementia Rating score of 1, Mini Mental State Exam score of 20 to 27, and CSF amyloid positivity.
Participants were randomized 1:3 to receive placebo or BIIB080 at low, medium, and high doses. Researchers administered drug or placebo via intrathecal injection every four weeks for three months, followed by a six-month monitoring period (see image below). One group received two high doses three months apart. Once the monitoring period wrapped up, everyone began receiving the ASO at his or her assigned dose every 13 weeks for another year with up to six months of monitoring afterwards. This open-label extension (OLE) is expected to wrap up in early 2022.
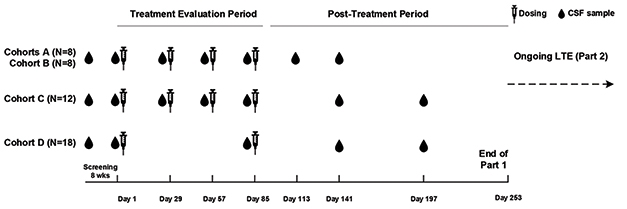
Dosing and Testing. Participants received low (cohort A), medium (cohort B), and high (cohorts C and D) doses of BIIB080 over a three-month treatment period. That was followed by a six-month sampling period. [Courtesy of Catherine Mummery, University of College London.]
The primary outcome measure was adverse events, while secondary outcomes included CSF tau and pharmacokinetics. No participants had serious adverse events and 43 have completed the post-OLE monitoring. Three-quarters of the placebo group reported at least one side effect, as did 94 percent of those who received the drug. Up to 30 percent of the treatment group experienced mild to moderate effects, such as headache, pain while the drug was being injected, or post-lumbar puncture syndrome (see image below). The latter occurs if the punctured dura mater leaks CSF, dropping intracranial pressure and causing a characteristic posture-dependent headache (Cohen et al., 1991).

Painful for Some. A majority of participants in both placebo and treatment groups reported mild side effects, which the researchers attributed to the lumbar puncture procedure itself. [Courtesy of Catherine Mummery, University College London.]
Did the drug hit its target? CSF total tau and phospho-tau181 levels fell over time, dropping up to 35 percent in the low-dose group and up to 50 percent in the high-dose group eight weeks after the last dose (see image below). In people who received the high dose, the markers continued dropping over the next eight weeks, falling another 5 percent.
Other tau immunotherapies currently in trials, such as anti-tau antibodies, focus on extracellular tau, nipping its spread and aggregation in the bud. In contrast, ASOs go after expression, which can quash tau levels inside and outside of cells. “Knocking down all tau is a more exciting and logical approach to me, given our uncertainty about which isoform is pathological,” Mummery told Alzforum. Boxer agreed. “Even if the hypothesis that prion-like spread is important for tauopathies is not correct, genetically suppressing tau with ASOs will give us a much better idea of whether tau is central to neurodegeneration,” he told Alzforum. Rabinovici noted that slashing all tau may make this approach applicable to multiple tauopathies, since each involves different oligomers and fibril conformations.
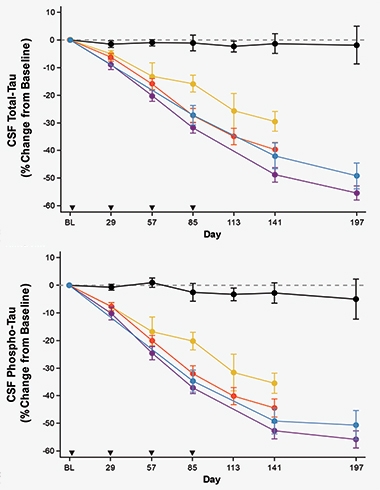
Tumbling Tau. Both total tau (top) and p-tau181 (bottom) dropped in the CSF of participants treated with low (yellow), medium (red), or high (blue and purple) dose BIIB080, while tau in people given placebo (black) remained unchanged. Arrowheads indicate when the drug or placebo were given. [Courtesy of Catherine Mummery, University of College London.]
But what do lower CSF tau levels mean clinically? For now, we do not know. “The strong dose relationship shows a real biological effect, but the big question is whether that translates into clinical benefits,” Mummery told Alzforum. The anti-tau antibodies semorinemab and gosuranemab also lowered tau in the CSF but had no clinical benefit in AD (May 2021 news; Jun 2021 news).
Ditto for Ionis’ ASO for Huntington’s disease. Tominersen, also called HTTRx, halved mutant Htt levels in CSF, but provided no clinical improvement for patients. A Phase 3 trial screeched to a halt this March (Mar 2018 news; company press release).
“The pathological mechanisms are unique among neurodegenerative disorders, so it is difficult to compare ASOs across indications,” Junge wrote to Alzforum. Rabinovici agreed. “The success of one ASO may, or may not, predict the success of others,” he said.
Although it is still early days, Boxer is hopeful for BIIB080, noting that when it lowered tau in the CSF of nonhuman primates, levels of tau fell in their hippocampi as well, as judged by postmortem analysis (Jan 2017 news; DeVos et al., 2017). Mummery agreed. “The quality of the preclinical work is one of the reasons I am very excited about this drug's potential,” she said.
Eric Reiman, Banner Alzheimer’s Institute, Phoenix, wondered how the ASO affects other fluid markers. “Additional biomarker data, including for downstream neurodegenerative biomarkers such as NfL, and clinical data will be needed to clarify the treatment’s disease-slowing effects,” he wrote to Alzforum (full comment below).
Rabinovici raised a concern about how reducing tau low enough to see pathological changes relates to cognition. “With BACE inhibitors, we did not know that aggressive inhibition would hasten cognitive decline until late-stage trials, so how much tau lowering is too much?” he asked.
A Phase 2 trial of BIIB080 for mild AD is planned. “While I haven’t seen the protocol yet, trial sites have been approached to participate and we hope it will begin by mid-2022,” Mummery told Alzforum. She believes the trial will include U.S. and European sites.—Chelsea Weidman Burke
References
Therapeutics Citations
News Citations
- Positive Trials of Spinal Muscular Atrophy Bode Well for Antisense Approach
- As RNA Therapies Come of Age, Efficacy Remains Weak
- ASOs: Wave of the Future in Alzheimer’s Therapeutics?
- Anti-Tau Antibody Data Leave Best Isotype Unclear
- Biogen Shelves Gosuranemab After Negative Alzheimer’s Trial
- Antisense Therapy Cuts Huntingtin Protein in CSF by Half
- Antisense Oligos Tango with Tau Transcripts to Reverse Tauopathy
Paper Citations
- Cohen Y, Pouchot J, Vinceneux P. [Lumbar post-puncture syndrome]. Rev Med Interne. 1991 Nov-Dec;12(6):429-32. PubMed.
- DeVos SL, Miller RL, Schoch KM, Holmes BB, Kebodeaux CS, Wegener AJ, Chen G, Shen T, Tran H, Nichols B, Zanardi TA, Kordasiewicz HB, Swayze EE, Bennett CF, Diamond MI, Miller TM. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. 2017 Jan 25;9(374) PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Arizona Alzheimer's Consortium
Gene-silencing treatments like anti-sense oligonucleotides (ASO) and RNAi’s, and the other gene-editing technologies and treatment delivery systems that will follow in the coming years, have extraordinary promise for the treatment and prevention of AD. This important Phase 1B multiple-ascending-dose study evaluated the safety, tolerability, and CSF tau-reducing effects of high, moderate, and low monthly intrathecal bolus doses and a high, every-three-month dose of ISIS 814907 (aka BIIB080) compared to placebo in 46 CSF-biomarker-positive patients in the early clinical states of AD. Patients were treated for three months and followed for an additional six months. Besides the procedure discomfort and headaches that followed the lumbar punctures, the treatment appeared to be safe and well-tolerated in this small initial study. Importantly, there were progressive dose-dependent reductions in CSF total tau and CSF p-tau measurements, averaging more than 50 percent reductions after nine months in the highest monthly and every-three-month doses.
This study supports the ability of this microtubule-associated tau protein gene-silencing treatment to suppress neuronal tau production in patients with AD and it provides initial safety data to support the feasibility of this innovative approach to the treatment of tau pathology. While this study provides evidence of target engagement, additional biomarker data, including those that evaluate downstream neurodegenerative biomarkers such as NfL, and clinical data will be needed to clarify the treatment’s disease-slowing effects, and additional data will be needed to provide information about its long-term safety and tolerability. That data will come initially from a long-term extension study and, depending on the initial findings, studies in larger groups.
We were reminded of the potentially transformational impact of gene therapies following the use of an ASO for spinal muscular atrophy. We were also reminded that there may also be challenges to the use of ASO’s in the recent studies of Huntington’s disease, which failed to demonstrate a significant clinical benefit, despite similar suppression of the wild-type protein implicated in the disease, and raised the possibility of slightly deleterious effects at higher doses. Despite that recent failure, and the potential explanations for the disappointing results, such as depth of tissue penetration, adaptive roles of the suppressed wildtype protein, etc., we look forward to seeing approaches like this being put to the test in patients with this devastating disease.
I congratulate the authors for this important work, their exciting preliminary findings, and the clear way in which they have presented their preliminary results.
Make a Comment
To make a comment you must login or register.