BAN2401 Forges AHEAD into Phase 3, Preclinical AD
Quick Links
Part 2 of 3
As data from Phase 2 open-label extension studies is trickling in (see Part 1 of this story), the true test of BAN2401’s efficacy in both the early and preclinical stages of Alzheimer’s disease begins. In presentations at the virtual CTAD conference, held November 4-7, researchers described the population recruited into CLARITY-AD, Eisai and Biogen’s Phase 3 trial of BAN2401 in people at the earliest symptomatic stages of AD. This trial started in March 2019 and is nearing full enrollment. Meanwhile, AHEAD 3-45—a paired Phase 2 and Phase 3 study testing the antibody in two preclinical stages of AD—has started recruiting. This secondary prevention trial is headed by the Alzheimer’s Clinical Trial Consortium, in collaboration with Eisai and Biogen.
At CTAD, Eisai’s Shau Yu Lynch updated viewers on the status of CLARITY-AD, including baseline characteristics of the participants enrolled so far. In contrast to its more complicated adaptive predecessor, this trial follows a straightforward parallel group design, randomizing half of participants to receive biweekly infusions of placebo, or 10 mg/kg BAN2401, over 18 months. Change in the CDR-SB between baseline and 18 months serves as the primary endpoint, with other cognitive measures included among secondary endpoints. In addition, the trial will track MRI, amyloid and tau-PET scans, and CSF and plasma biomarkers including Aβ42, neurogranin, NfL, total and p-tau, in a subset of participants throughout the trial. Those who complete the trial can join an open-label, two-year extension that will continue to monitor imaging and fluid biomarkers and cognition.
To date, the trial has enrolled 1,222 of its projected 1,500 participants. At baseline, the randomized cohort is quite similar to that of the Phase 2 study, Lynch reported. Participants average 72 years of age, just over half are women, and their baseline cognitive scores reflect their early symptomatic stage of AD. Seventy-six percent are white, 18 percent Asian, 3 percent black. Sixteen percent of the cohort is of Hispanic or Latino ethnicity.
Based on data from the Phase 2 trial and ongoing OLE, the researchers expect one in 10 participants in the treatment arm of this Phase 3 trial to develop ARIA-E. Rather than automatically discontinue dosing in those patients, they will monitor their ARIA-E and continue dosing in those whose ARIA-E is mild to moderate, based on the radiographic severity of the abnormality on MRI.
In the wings of this symptomatic trial, AHEAD 3-45, the ambitious pair of secondary prevention trials, is now ramping up. Reisa Sperling of Harvard Medical at CTAD presented the design and early imaging data on the first enrollees.
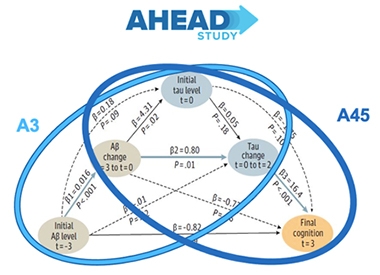
Sister Studies. A3 and A45 target different stages of preclinical AD. A3 aims to nip amyloid accumulation in the bud, before it triggers tau accumulation. A45 targets amyloid in people who already have a substantial plaque burden, aiming to stop cortical tau and fend off cognitive decline. [Slide courtesy of Reisa Sperling.]
Essentially, AHEAD 3-45 comprises sister studies, each trying to treat a different stage of preclinical AD. Participants—all of whose cognition is still normal—are placed into A3 or A45 based on their amount of amyloid accumulation as measured with PET scans. Those whose brain amyloid levels fall between 20 and 40 centiloids—below the threshold of brain-wide positivity as per a visual read—enter A3, while those who post above 40 centiloids enter A45.
Researchers will track the same cognitive, biomarker, and imaging measures in both trials. However, the primary outcomes differ between the two. A3, a Phase 2 study, aims to enroll 400 participants and will use their change in amyloid-PET as its primary endpoint. A45, a Phase 3, will enroll 1,000 volunteers and use change in their performance on the preclinical Alzheimer’s cognitive composite version 5 (PACC5) as its primary. Both trials are slated to run for 216 weeks—aka, a whopping four years.
The AHEAD 3-45 selection process relies heavily on amyloid-PET imaging. The goal for A3 is to nip amyloid accumulation in the bud during its early phase of rapid acceleration, in hopes of forestalling subsequent tangle formation. For A45, by contrast, the aim is to aggressively remove amyloid and prevent an all-out “ca-tau-strophy,” Sperling said, whereby tau pathology spreads broadly into the neocortex and cognition starts to decline.
To set the amyloid cutoffs for each trial, the researchers drew upon large longitudinal datasets including the Alzheimer’s Disease Neuroimaging Initiative (ADNI), Harvard Aging Brain Study (HABS), and the Australian Imaging, Biomarker, and Lifestyle (AIBL) study (Feb 2020 news).
Given the distinct goals of the two trials, they will use different dosing regimens. Based on data from BAN2401’s Phase 2 study, a monthly dose of 10 mg/kg BAN2401 may be sufficient to reduce amyloid below a critical threshold of 20 centiloids in people who start the trial with intermediate amyloid levels, i.e., those in A3. Those who start out with more amyloid, in A45, will receive this amount twice a month.
To reduce odds of ARIA-E, both trials will start with a titration period, ramping up to their respective target doses over eight weeks. Finally, volunteers in A45 will move onto a lower, monthly maintenance dose after 60 weeks. To mitigate danger posed by the COVID-19 pandemic, infusions will be given in participants’ homes, Sperling said. The trialists also plan to incorporate remote assessments whenever possible, though key cognitive tests, including the PACC5, need to be conducted in the clinic.
Sperling emphasized that the trial is using NAV4694 to measure amyloid for screening as well as monitoring throughout both trials. NAV4694 is a superior PET tracer that was stuck in development limbo for years and has only recently become available (May 2010 conference news; Oct 2014 news). The tracer rivals the sensitivity of PiB, yet uses the more stable fluorine 18 (F18) radioisotope instead of PiB’s ephemeral C11. Sperling believes the greater sensitivity of this tracer will prove essential to accurately gauge low levels of amyloid, and changes in those levels with treatment (Therriault et al., 2020).
What do these participants look like? Sperling showed amyloid imaging data from the first 33 who were scanned during screening. As predicted from past studies, the researchers found that about half, i.e. 16, were amyloid-negative. Among the other half, three fell into the intermediate range of 20-40 centiloids, while the remaining 14 had obvious accumulation above 40 centiloids. Sperling declined to say how many participants had already enrolled, but said that the first patient was randomized to A45 in September, and to A3 the first week of November.
At four years in duration, AHEAD 3-45 is a long haul. Its length is necessary to tease out a treatment benefit in people in the preclinical stage of the disease, where change comes slowly. With the pitfalls of aducanumab’s clinical path duly noted, no interim futility analyses are planned for AHEAD3-45, Sperling said. However, the scientists do plan to conduct an interim analysis at 96 weeks. At that point, they will take stock of cognitive and biomarker data from both trials. Only if the findings were to be “wildly positive” would the researchers consider halting the blinded portion of the trial and moving all participants into an open-label extension, Sperling said. Similarly convincing data from CLARITY-AD, which will finish before AHEAD3-45, could also influence this decision, she added.
Where do all these preclinical patients come from? For that, see Part 3 of this story.—Jessica Shugart
References
News Citations
- BANish Aβ? BAN2401 Antibody Makes Its Move in Phase 3 Program
- How Much Amyloid Will Kick Off Tangles, and Decline?
- Geneva: The AstraZeneca Ligand—The Fairest of Them All?
- Lilly Teams Up With AstraZeneca for BACE Inhibitor Phase 2/3 Trial
- TRC-PAD Funnel Finally Touches Down
Paper Citations
- Therriault J, Benedet AL, Pascoal TA, Savard M, Ashton NJ, Chamoun M, Tissot C, Lussier F, Kang MS, Bezgin G, Wang T, Fernandes-Arias J, Massarweh G, Vitali P, Zetterberg H, Blennow K, Saha-Chaudhuri P, Soucy JP, Gauthier S, Rosa-Neto P. Determining Amyloid-β Positivity Using 18F-AZD4694 PET Imaging. J Nucl Med. 2021 Feb;62(2):247-252. Epub 2020 Jul 31 PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.