Focused Ultrasound Breaches Blood-Brain Barrier in People with Alzheimer’s
Quick Links
When seduced by microbubbles jiggling to the beat of ultrasonic waves, even the uptight blood-brain barrier lets its guard down. That’s according to the first study to target the barrier noninvasively with focused ultrasound. Researchers used the strategy to temporarily ease open the blood-brain barrier in the prefrontal cortices of five people with Alzheimer’s disease. Led by Kullervo Hynynen and Sandra Black of the Sunnybrook Research Center in Toronto, the scientists propose that this strategy might allow drugs to cross into the brain. The findings, presented at the Alzheimer’s Association International Conference in Chicago July 22–26, were published July 25 in Nature Communications. Studies on animal models presented at the meeting supported the benefit of ultrasound, reporting it allowed experimental therapies, ranging from small molecules to antibodies, to access the brain to clear tau, protect dopaminergic neurons, and boost neurogenesis.
- Ultrasound pulses opened the human blood-brain barrier.
- The barrier closed again within 24 hours, and no adverse events occurred.
- In animals, ultrasound boosted neurogenesis and synaptic function, and helped clear Aβ plaques and tau tangles.
Designing drugs that can transit the blood-brain barrier (BBB) is a major hurdle drug developers struggle to cross. Nearly two decades ago, researchers discovered that low-energy ultrasound waves could temporarily loosen the barrier (Hynynen et al., 2001). Just prior to applying the sound waves, researchers inject microbubbles into the blood. These 1–10 micron diameter chambers of inert gas line the walls of capillaries and larger blood vessels, including those of the BBB. When hit with ultrasound, the microbubbles expand and contract, and this movement loosens the tight junctions between blood vessel cells that cement the barrier. Multiple studies in animals have demonstrated that the ultrasound/microbubble combo temporarily holds the door open for large molecules, such as antibodies, to sneak across (Samiotaki et al., 2015; Nov 2009 conference news). What’s more, researchers have reported that simply opening the barrier has its own benefits, including clearance of Aβ plaques in AD mouse models via activation of microglia (Choi et al., 2008; Burgess et al., 2014; Mar 2015 news).
Does ultrasound open the BBB in people? And is that safe? Those were the questions first author Nir Lipsman and colleagues set out to answer. They enrolled three men and two women with mild to moderate AD who had tested positive for Aβ plaques in the brain on 18F-florbetaben PET scans. They averaged 66 years old. The volunteers received two rounds of focused ultrasound, spaced one month apart. After injecting microbubbles intravenously, the researchers delivered the sound waves in short pulses for up to a minute at an average power of 4.5 watts. This is less than 1 percent of the ultrasound energy used to surgically ablate tissue, such as uterine fibroids or tumors. MRI scans were used to target the sound waves to a small region in the dorsolateral prefrontal cortex. The primary outcome measure of the study was BBB opening, which was measured by tracking diffusion of the MRI contrast agent gadolinium into the brain.
The scientists targeted the waves to a 5mm-by-5mm region for the first round, then doubled the target area for the second round. One patient developed a respiratory infection and was not given the second round of focused ultrasound (FUS), because the researchers did not want to open his BBB during an active infection.
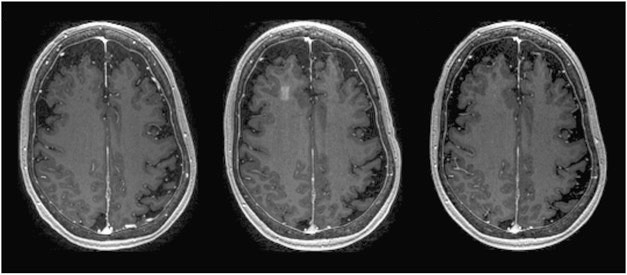
Tiny Door into the Brain. MRI of one patient reveals infiltration of gadolinium contrast in the right frontal lobe immediately after focused ultrasound (middle). The opening is not present before (left) or 24 hours after (right) the ultrasound.
Immediately following the ultrasound, Lipsman observed a discrete infiltration of gadolinium in the targeted region, suggesting disruption of the BBB. One day later, the researchers injected gadolinium again but saw none seep into the brain, suggesting the barrier had resealed. All patients went home the day after the procedure, and none reported any adverse events. According to MRIs, FUS did not trigger hemorrhages or brain swelling, but the researchers did find tiny, round, white-matter hypointensities in the targeted area in two patients immediately following FUS. These hypointensities had disappeared the following day. Lipsman believes they were not microhemorrhages, which would have been likely to persist for more than 24 hours.
The safety findings were welcome news to Cynthia Lemere of Brigham and Women’s Hospital in Boston, who co-chaired the ultrasound session at AAIC. Lemere told Alzforum that a few years ago, the idea of opening the blood-brain barrier in people with AD, who tend to have vascular problems on top of Aβ deposits, sounded risky and counterintuitive. Now this human safety data, together with experiments she and other researchers have done in aged AD mice, are beginning to make the strategy seem promising, she said.
In the small number of people studied thus far, Lipsman and colleagues also looked for changes in amyloid, cognition, and daily functioning. They found no differences between baseline and three months following the second round of ultrasound.
Lipsman told Alzforum that a Phase 2a FUS study is slated to begin in September. It will enroll 30 participants and target larger regions of the brain, including those known to accumulate the most Aβ plaques. He added that if the ultrasound procedure can be established to be safe, then it could potentially be used with any therapeutic that does not easily cross the BBB.
Animal Models Suggest Benefits
Researchers have been studying this approach in different animal models of neurodegeneration. At AAIC, Elisa Konofagou of Columbia University in New York reported that focused ultrasound helped deliver neurotrophic factors into the brain in a mouse model of Parkinson’s disease. First, Konofagou presented findings from the MPTP injection model, in which dopaminergic neurons wither following administration of this neurotoxin. After injecting microbubbles into the blood, she targeted sound waves to the substantia nigra and striatum, the areas most prone to degeneration in PD. She then injected either saline or the neurotrophic protein neurturin, or an adeno-associated virus expressing glial-derived neurotrophic factor (GDNF). Other mice were given these treatments without ultrasound. By the time of treatment, about 40 percent of the dopaminergic neurons in the substantia nigra had died. Konofagou reported that when given together with ultrasound, either growth factor restored about 75 percent of what was lost, and corrected motor deficits the mice had developed by that time.
Konofagou also used the strategy to open a door to the brain for α-synuclein antibodies. She reported that ultrasound significantly enhanced the delivery of the antibodies to the brains of six-month-old A53T and A30T mice, which express mutant forms of human α-synuclein that cause PD. In both cases, the antibodies strongly reduced α-synuclein pathology in animals that had received ultrasound to open the BBB.
Previously, researchers led by Hynynen and Isabelle Aubert at the Sunnybrook Research Institute in Toronto reported that in the TgCRND8 mouse model of amyloidosis, ultrasound alone activated microglia, stimulating them to remove Aβ plaques (Jordão et al., 2013). However, Konofagou told Alzforum that ultrasound neither activated microglia nor reduced α-synuclein deposits in the PD models. Perhaps microglia are primed differently in the presence of amyloid, she speculated. Jürgen Götz of the University of Queensland in Brisbane agreed. He added that extracellular Aβ plaques—as opposed to intracellular α-synuclein inclusions—might be more amenable to clearance by activated microglia.
Götz had previously reported that merely opening the blood-brain barrier—sans therapeutics—motivated microglia to mop up Aβ plaques, in this case in APP23 mice (Mar 2015 news). Götz used scanning ultrasound, in which the ultrasound waves are delivered in short pulses throughout the entire brain. However, this was less efficient in clearing tau pathology, he found. It did aid the delivery of RN2N, a single-chain, tau-specific antibody fragment, into the brains of P301L mice, where the fragment made its way into neurons and dendrites. The combination treatment reduced phospho-tau more than the antibody alone did (Nisbet et al., 2017).
At AAIC, Götz extended these findings to a model of frontotemporal dementia with parkinsonism. K3 mice express the K369I mutant of tau in the substantia nigra. They rapidly develop neurofibrillary tangle pathology and an early onset parkinsonian motor phenotype, and anti-tau antibodies reduce their phospho-tau and tangles but not their motor problems (Ittner et al., 2008; Ittner et al., 2015). At AAIC, Götz showed that if the BBB was repeatedly opened with weekly scanning ultrasound beginning at five weeks of age, not only did the mice accumulate less phospho-tau and tau tangles than controls, but their motor coordination, as tested by the Rotarod test, improved after the fifth sonication. This suggested that when given enough times, scanning ultrasound alone is beneficial.
“We have done multiple studies with these mice in the past, and it was almost impossible to see improvement in motor symptoms with an antibody alone,” he told Alzforum. “Now, using ultrasound alone, we finally see real improvement.”
Taking a different tack, Aubert has used focused ultrasound to promote adult neurogenesis and neuronal survival, which wane as AD progresses. Previously, Aubert and colleagues reported that temporarily easing the BBB with focused ultrasound stimulated adult neurogenesis in the hippocampus (Scarcelli et al., 2014; Mooney et al., 2016). At AAIC, she reported that adding a pro-survival molecule into the mix boosted neurogenesis further. Specifically, Aubert treated TgCRND8 mice with ultrasound alone or in combination with a small molecule agonist of tropomyosin receptor kinase A (TrkA), a high-affinity receptor for neurotrophin. Ultrasound alone triggered pro-survival responses, such as MAP kinase activation, but adding the TrkA agonist dramatically enhanced them. The same was true in non-transgenic mice, suggesting that the beneficial effects of the agonist were not dependent on Aβ pathology.
Aubert has also used ultrasound to deliver intravenous immunoglobulins (IVIg) into the brains of TgCRND8 mice. IVIg, a mixture of antibodies pooled from blood donors, has been tested as a potential therapy for AD but failed in Phase 3 (May 2013 news). The rationale was that anti-Aβ and other antibodies might promote beneficial immune responses, clear Aβ, and restore brain function. At AAIC, Aubert reported that FUS facilitated the delivery of immunoglobulin from the mixture into the brains of TgCRND8 mice; alas, 20 days later, the researchers were surprised to find that the FUS had not promoted more clearance of Aβ than did IVIg alone, but rather had enhanced neurogenesis.
Aubert proposed that cytokine changes brought about by both the ultrasound and IVIg somehow influenced the birth rate of newborn neurons. As to which cells are expressing those cytokines, Aubert reported activation and proliferation of microglia in response to ultrasound. Lemere pointed out that the complement pathway, which is upregulated in activated glial and myeloid cells, might play a role in promoting neurogenesis in response to ultrasound. She added that it may also be possible that circulating monocytes gain access to the brain while the BBB is open. Konofagou agreed that infiltration of monocytes was a possibility. Both researchers agreed that if, and how, peripheral cells mediate ultrasound effects need further study.—Jessica Shugart
References
News Citations
- Chicago: New Technologies Help Drugs Cross Blood-Brain Barrier
- Stop, Hey, What’s That Sound? ... Amyloid Is Going Down?
- Gammagard™ Misses Endpoints in Phase 3 Trial
Research Models Citations
Therapeutics Citations
Paper Citations
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001 Sep;220(3):640-6. PubMed.
- Samiotaki G, Acosta C, Wang S, Konofagou EE. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood-brain barrier opening in vivo. J Cereb Blood Flow Metab. 2015 Jan 14; PubMed.
- Choi JJ, Wang S, Brown TR, Small SA, Duff KE, Konofagou EE. Noninvasive and transient blood-brain barrier opening in the hippocampus of Alzheimer's double transgenic mice using focused ultrasound. Ultrason Imaging. 2008 Jul;30(3):189-200. PubMed.
- Burgess A, Dubey S, Yeung S, Hough O, Eterman N, Aubert I, Hynynen K. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology. 2014 Dec;273(3):736-45. Epub 2014 Sep 15 PubMed.
- Jordão JF, Thévenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, O'Reilly M, Huang Y, McLaurin J, Hynynen K, Aubert I. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol. 2013 Oct;248:16-29. Epub 2013 May 21 PubMed.
- Nisbet RM, Van der Jeugd A, Leinenga G, Evans HT, Janowicz PW, Götz J. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain. 2017 Mar 4; PubMed.
- Ittner LM, Fath T, Ke YD, Bi M, van Eersel J, Li KM, Gunning P, Götz J. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15997-6002. Epub 2008 Oct 2 PubMed.
- Ittner A, Bertz J, Suh LS, Stevens CH, Götz J, Ittner LM. Tau-targeting passive immunization modulates aspects of pathology in tau transgenic mice. J Neurochem. 2015 Jan;132(1):135-45. Epub 2014 Aug 1 PubMed.
- Scarcelli T, Jordão JF, O'Reilly MA, Ellens N, Hynynen K, Aubert I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014 Mar-Apr;7(2):304-7. Epub 2014 Jan 28 PubMed.
- Mooney SJ, Shah K, Yeung S, Burgess A, Aubert I, Hynynen K. Focused Ultrasound-Induced Neurogenesis Requires an Increase in Blood-Brain Barrier Permeability. PLoS One. 2016;11(7):e0159892. Epub 2016 Jul 26 PubMed.
Further Reading
Papers
- Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol. 1995;21(7):969-79. PubMed.
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001 Sep;220(3):640-6. PubMed.
- Konofagou EE. Optimization of the ultrasound-induced blood-brain barrier opening. Theranostics. 2012;2(12):1223-37. Epub 2012 Dec 31 PubMed.
- Alonso A. Ultrasound-induced blood-brain barrier opening for drug delivery. Front Neurol Neurosci. 2015;36:106-15. Epub 2014 Dec 22 PubMed.
- Morrone CD, Thomason LA, Brown ME, Aubert I, McLaurin J. Effects of Neurotrophic Support and Amyloid-Targeted Combined Therapy on Adult Hippocampal Neurogenesis in a Transgenic Model of Alzheimer's Disease. PLoS One. 2016;11(10):e0165393. Epub 2016 Oct 21 PubMed.
Primary Papers
- Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, Smith GS, Hynynen K, Black SE. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. 2018 Jul 25;9(1):2336. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.